Asas Nsaid Index
Dosages of NSAIDs accounted by the ASAS NSAID index. 13112018 Patients failing a trial of 2 different NSAID each for at least 2 weeks with optimum dosage without response or with partial response and Bath Ankylosing Spondylitis Disease Activity Index BASDAI score of 4 range 0-10 or Ankylosing spondylitis disease activity score-C reactive proteinASDAS-CRP21 will be considered as primary entry criteria for this study.

Changes In Nsaid Use At Group Level Download Table
2014 Dougados et al.

Asas nsaid index. In the present study we compared ASAS HI between patients with ankylosing spondylitis AS and those with nonradiographic. At baseline 6 pts received TNF inhibitors after 2 y. 01022011 For the NSAID equivalent scoring system the recommendations are 1 to refer to a scale in which 0 no intake 100 150 mg diclofenac 1000 mg naproxen 200 mg aceclofenac 400 mg celecoxib 600 mg etodolac 90 mg etoricoxib 200 mg flurbiprofen 2400 mg ibuprofen 150 mg indometacin 200 mg ketoprofen 15 mg meloxicam 400 mg phenylbutazone 20 mg piroxicam 20 mg tenoxicam.
05042019 PDF The Assessment of Spondyloarthritis International Society ASAS health index HI is a novel tool for approaching disability health and. Carbo1 Anneke Spoorenberg12 Fiona Maas1 Elisabeth Brouwer1 Reinhard Bos2 Hendrika Bootsma1 Eveline van der Veer3 Freke Wink2 Suzanne Arends12 1 Rheumatology and Clinical Immunology University Medical Center Groningen University of Groningen. In clinical practice nonsteroidal anti-inflammatory drugs NSAIDs are commonly discontinued after response to biologic therapy is achieved in patients with axial spondyloarthritis axSpA but the impact of NSAID discontinuati.
The self-reprt questionnaire measures functioning and health. It combines five disease activity variables with only partial overlap resulting in one single score with better truth validity enhanced discriminative capacity and improved sensitivity to change as compared to single-item variables 12. The Ankylosing Spondylitis Disease Activity Score ASDAS is a new composite index to assess disease activity in Ankylosing Spondylitis AS1.
No significantly differences in axSpA disease activity between two groups at baseline table 1 and after 2. FUP 14 pts receives TNF inhibitors. ASAS NSAID Intake Score for Use in Clinical Trials Epidemiological Studies of Spondyloarthritis.
R Akkoc N Brandt J Chou CT Dougados M Huang F Gu J Kirazli Y Van den Bosch F Olivieri I Roussou E Scarpato S Srensen IJ Valle-Oate R. 24022016 To evaluate the early effect of fulldose nonsteroidal antiinflammatory drugs NSAIDs on the extent and intensity of bone marrow edema of the sacroiliac SI joints on magnetic resonance imaging MRI. Similarly there was significant reduction in mean ASAS NSAID index from 296 to 14 over 6 months from baseline p 0001 and it was similar in both groups.
Multivariable regression analysis of the axSpA group showed that high NSAID intake and mSASSS were positively associated with ASAS HI whereas higher economic status and alcohol consumption were negatively associated with ASAS HI. Ann Rheum Dis 201170249-51Toll-Free Link Rudwaleit M van der Heijde D Landew. AB0688 Effect of treatment with non-steroidal anti-inflammatory drugs on disease activity in patients with early axial spondyloarthritis base on data from 2 years follow up of corsar cohort.
29122019 NSAID use by means of the ASASNSAID score 0100. The ASAS Health Index has been developed under the auspices of the Assessment of SpondyloArthritis international Society ASAS to assess health in patients with all forms of spondyloarthritis SpA specifically radiographic and non-radiographic axial SpA as well as peripheral SpA. 07012021 ASAS recommendations for collecting analysing and reporting NSAID intake in clinical trialsepidemiological studies in axial spondyloarthritis.
17082019 related to NSAID use especially in patients treated with TNF-α inhibitors Marlies J. Marlies J G Carbo Anneke Spoorenberg Fiona Maas Elisabeth Brouwer Reinhard Bos Hendrika Bootsma Eveline van der Veer Freke Wink Suzanne Arends. At each follow-up visit approximately half of the patients changed their type or dose of NSAIDs.
Ankylosing spondylitis disease activity score is related to NSAID use especially in patients treated with TNF-alpha inhibitors. ASAS-NSAID index also decreased significantly from median 65 to 0. In patients on conventional treatment n 139 74 used NSAIDs at baseline with median ASAS-NSAID index of 50 and this remained stable during follow-up.
Bath Ankylosing Spondylitis Disease Activity Index BASDAI BASDAI is a selfadministered questionnaire to assess disease activity using a visual analog scale VAS. ASAS HI correlated with other SpA-related parameters such as BASDAI ASDAS and BASFI. Using BASDAI 4 to define active disease a 34 reduction in requirement of biologicals was also observed.
2 to present the results as mean daily intake by considering the number of days on which NSAID. The Assessment of Spondyloarthritis International Society ASAS health index HI is a novel tool for approaching disability health and functioning in spondyloarthritis SpA. Arthritis Research and Therapy.

Asas Classification Criteria For Axial Spondyloarthritis Published Download Scientific Diagram
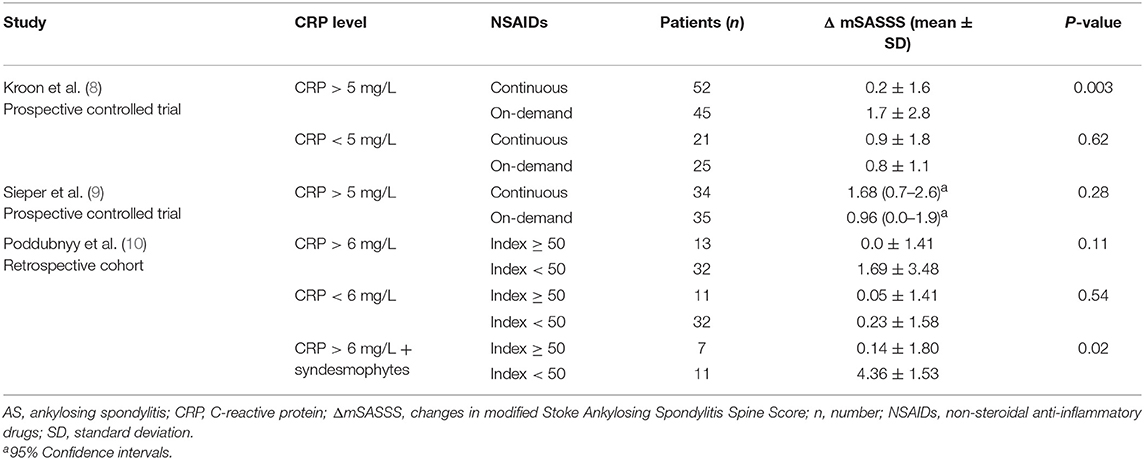
Frontiers Non Steroidal Anti Inflammatory Drugs Are Unlikely To Inhibit Radiographic Progression Of Ankylosing Spondylitis A Systematic Review Medicine
Comments
Post a Comment